Organic synthesis with Thermo Scientific chemicals

Explore our reagents and materials for synthesis and study of reaction mechanisms, including air- and moisture-sensitive reagents and building blocks.
Our extensive collection of chemicals and reagents enable innovation, increase productivity and improves laboratory safety. We offer choice, quality and supply assurance. We have an established, robust global sourcing network that offers the convenience of pre-packed catalogue products in several sizes, as well as custom chemical ranges that provide flexible solutions for chemistry workflows.
Ask for a poster
With our extensive portfolio of chemicals and our expertise, we can offer comprehensive solutions to meet all your chemical needs for your research, pilot-scale or production application. Whether you're conducting initial research or scaling up to full production, we can tailor our products exclusively for you. Our bulk chemical solutions empower you to go beyond the catalog, enabling seamless scaling from grams to hundreds or even thousands of kilograms.
Experience end-to-end support with our custom and bulk chemical services.
- Custom synthesis: Save time and improve product consistency by letting us prepare your custom-made chemicals at our facilities
- Custom specifications: Take advantage of our extensive chemical services to define and meet your unique specifications
- Custom labeling: Use our labeling services to display the right product or batch details to enhance product identification for your laboratory
- Custom packaging & pack size: Get the right product in the packaging material or size that you require
- Bulk chemicals: Our bulk chemical solutions will allow you to go beyond the catalog and scale up from grams to hundreds or thousands of kilograms
Functional Reagents
The most commonly used functional reagents, plus more unusual reagents for specific reactions.
Heterocyclic Compounds
Heterocyclic compounds are cyclic compounds containing atoms from at least two different elements in the ring, and include nitrogen, oxygen, sulphur and mixed heterocycles.

Hydrocarbons
Hydrocarbons are organic compounds made up entirely of carbon and hydrogen atoms.

Inorganics, Catalysis and Ligands
Inorganics include salts, metals, and substances made from single elements. In general, they are ionic compounds consisting of cations and anions joined by ionic bonding.

Halogen Containing Compounds
Organohalides are organic compounds containing fluorine, chlorine, bromine, and iodine.

Nitrogen Containing Compounds
Nitrogen-containing compounds include, among others, amines and derivatives, nitriles, azides, and hydrazines.
Oxygen Containing Compounds
Oxygen-containing compounds include alcohols, aldehydes, ketones and ethers, also carboxylic acids and derivatives.

Phosphorous Containing Compounds
Phosphorus-containing compounds include esters of organophosphorus acids, phosphonic esters, phosphines, and phosphites.

Sulfur Containing Compounds
Sulfur-containing compounds include thiols, thioethers, thioesters, thioureas, sulfonamides, and sulfoxides.

Materials Science and Polymers
Organic polymers include polysaccharides, polypeptides, polynucleotides, and synthetic materials such as the common plastics, e.g., polyethylene, polyvinylchloride, and polystyrene.
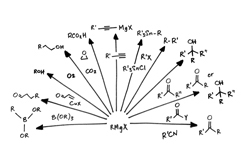
Organometallics
Chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon and selenium.

Biochemicals
Biochemical refer to any chemical that is found in a biological system or that can be used for biological research. Biochemical compounds include molecules such as amino acids, vitamins, and nucleotides as well as organic and inorganic chemicals.

Additional information
These are substances, (usually inorganic compounds or small organic molecules), used in the synthesis of organic compounds.