Organic synthesis with Thermo Scientific chemicals

Find compounds for each step of your organic synthesis workflow, including a wide variety of Thermo ScientificTM chemicals, catalysts, and reagents to progress your research with:
• Appropriate starting materials
• Increasing yield
• Supporting scale up
Whether you’re performing structural analysis and confirmation by NMR or using qualitative techniques, our products will help you complete your synthesis workflow. Scroll through a selection of our products below, or discover the entire range using our search bar.
Request For A Bulk Quote Key Named Reaction Poster request Ask for a poster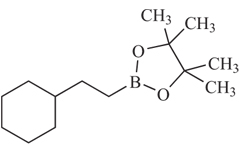
Building Blocks
Over 20,000 building blocks are available. A small selection of popular boronic acids and esters are listed here.

Organic Reagents
Our extensive range of functional and synthetic reagents, including air- and moisture sensitive products in industry-leading AcroSeal™ packaging.
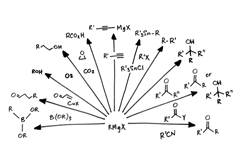
Organometallics
Wide-ranging organometallics including Grignard reagents, organolithium and organozinc compounds, with options for concentration, purity and pack size.
Catalysts
Providing an extensive range of catalysts, including precious metal compounds and homogeneous catalysts in varying purities and concentrations.

Essential Chemicals
Our complete range of ‘essentials’ – from solvents to drying agents, acids and bases, to help create the perfect environment and achieve highest yields.

Purification products
Providing purification products, including solvents, silica and alumina, when you need them.

Analytical products
For IR, UV and NMR spectroscopy, our broad range includes deuterated products with pack sizes, purities, standards and stabilisers to suit your requirements.