Chemical Safety In The Laboratory
When working with any chemicals, especially hazardous ones, safety is paramount. The appropriate personal protection equipment is, of course, mandatory but there are other options you might want to consider to help you keep your laboratory safe:
- Chemical handling – choosing products in easy to handle, safer containers can make all the difference
- Prepare for accidental breakage or spillages by having the appropriate absorbents to hand
- Avoid unnecessary hazards with safer chemical alternatives and monitoring of harmful residues
- Bench protection and safer dispensing
- Chemical storage including labelling of hazards
In addition, we’ve included some FAQs that we hope you will find useful plus information on REACH and CLP regulations. There is a download available of general safety guidelines and a technical guide plus links to our MSDS website search feature and product literature selector.
Watch the "Understanding chemicals to create a safer lab" webinar:
Safer Packaging Choices

Solvents and acids in plastic bottles
AnalaR NORMAPUR® acids and solvents in plastic bottles are safe, virtually unbreakable, environmentally friendly, light and easy to handle.

Coated glass bottles for acids
Safebreak bottles are specifically for acids. The PE-coating contains the acid if the glass is broken.

Aluminium bottles for HPLC solvents
Aluminium containers ensure solvent high purity as well as being unbreakable, light and easy to handle.

Safety bottle carriers
Colourful and practical safety carriers for the transport of acids, alkalis and solvents.
Chemicals for improving safety in the lab

Silica gels
While silica gels are safe dessicants, older moisture indicators that were included in them are not.

Biodegradable detergents
LABWASH® Premium concentrated detergents have high activity and low consumption biodegradable ingredients. Make one simple change to LABWASH® for excellent cleaning that is gentler on you and the environment.

Green solvents
Organic synthesis can involve toxic and carcinogenic substances. VWR Chemicals offer some greener alternatives solvents - Methyl-2 Tetrahydrofuran, 1,3 Dioxalane, Cyclopentyl methyl ether and 1,3 Propanediol that could reduce your exposure.
Anhydrous solvents
A complete range of high purity solvents with extremely low water levels specifically manufactured for moisture-sensitive organic and biotech applications.
Peroxide tests strips
Peroxide test strips are used to quickly identify inorganic peroxides in aqueous solutions and organic solvents.
Peracetic acid test strips
Peracetic acid test strips are used to identify any remaining peracetic acid after disinfection.
Products And Services For Your Safety

Spillage absorbents
You want to be able to contain any spills or breakages that occur quickly and efficiently, so have a selection of appropriate spillage absorbents to hand.
Safety labels
Safety labels are useful when you create a mixture or if you transport samples. They meet with GHS (Global Harmonised System) regulations for chemical substances and mixtures.

Bench top protectors
Bench protectors serve as an essential bench top barrier against corrosive spills, heated samples, or equipment vibrations.
Discover 15 common laboratory chemicals and how to arrange them
FAQ
ACIDS
1. What substances are incompatible with your acid?
- Acetic acid is incompatible with Chromium (IV) oxide, nitric acid, alcohols, ethylene glycol, perchloric acid, peroxides and permanganates
- Nitric acid is incompatible with acetic acid, aniline, chromium (IV) oxide, hydrocyanic acid, hydrogen sulphide, flammable liquids and gases
- Oxalic acid is incompatible with silver and mercury
- Perchloric acid is incompatible with acetic anhydride, bismuth and bismuth alloy, alcohols, paper and wood
- Sulphuric acid is incompatible with potassium chlorate, potassium perchlorate and potassium permanganate
2. Can I use citric acid GPR RECTAPUR® (20273*) to de-scale coffee machines?
YES! To de-scale a coffee machine, dissolve as much crystalline acid as possible in warm water (40 to 50 °C). When this solution is cool, decant it, leaving any undissolved sediment, and pour the solution, without the sediment, into the coffee machine. After descaling, rinse the coffee machine through once or twice with tap water. To be absolutely sure all the citric acid is gone, check the rinse water with a pH strip.
3. How can I safely work with hydrofluoric acid NORMATOM® (83873* or 85029*)?
A special safety combination for storing and dispensing hydrofluoric acid is available:
- Extra thick-walled bottle
- Specially designed safety cap and seal
- Special dispenser able to deliver the precise amount of acid you require ensuring the last drop is returned safely to the bottle
4. Managing explosion risks from perchloric acid 70% AnalaR® NORMAPUR® ACS ( 20589*)?
Highly concentrated perchloric acid is extremely corrosive, but not explosive. At normal pressure, it can be heated to boiling over a naked flame. However, if even the slightest trace of an organic impurity is present or if perchloric acid comes into contact with an organic substance, it may explode so careful storage and handling is needed to prevent contamination.
5. Why does VWR Chemicals supply concentrated nitric acid in plastic bottle with a short expiry date, 2 years?
In this time, concentrated nitric acid attacks plastic, with the result that the plastic bottle would eventually become brittle.
6. How can I safely work with highly concentrated formic acid 100% AnalaR® NORMAPUR® (20318*)?
Highly concentrated formic acid slowly breaks down into carbon monoxide and water. Gas formation raises the pressure in the bottle which may cause it to explode. The decomposition reaction accelerates at temperatures as low as 30 °C. Hence, we supply formic acid in bottles with special screw caps.
7. Why does VWR Chemicals supply nitric acid 99% (85851*, 85850*) with special packaging and special closures?
Fuming nitric acid gives off reddish -brown fumes owing to the action of light and warmth. These fumes cause the pressure inside the container to rise and, under certain circumstances, may cause the container to explode. VWR Chemicals special closure and packaging avoids these safety problems.
PACKAGING
8. What are the advantages and disadvantages of glass and high density polyethylene (HDPE) plastic bottles?
As well as increased safety from HDPE, these plastic bottles are useful when you use a lot of acids and/or are mindful of purchase costs. High quality of HDPE bottles ensure compatibility with our high purity analytical grade (Analar NORMAPUR) and the pack is cheaper and much lighter to handle than glass bottles.
9. What is EMBASAFE?
This special PE coated packaging affords maximum protection in the event of glass breakage as it contains any breakage making it easier to deal with. The glass is, of course, fully compatible with our high purity analytical grade (Analar® NORMAPUR®) and can be recycled the same as a classical glass bottle.
10. What is the storage life of substances in PE bottles?
Solvents: 5 years
Acids: 2 years
LABELS AND CLP REGULATIONS
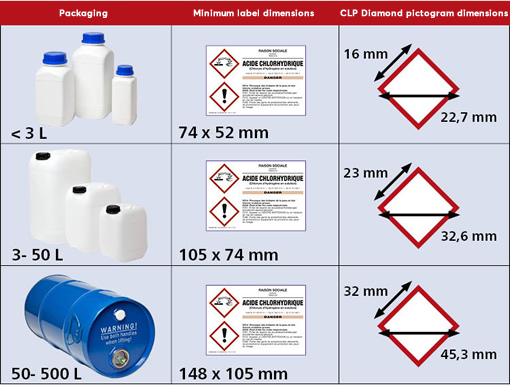
11. What are the minimum dimensions of labels for labelling your mixtures or samples?
12. How to read a VWR label?
The new multilingual labels are designed to help you find the information you need easily and quickly. They always include an expiry date for unopened and correctly stored product and usually a space for you to enter the date of receipt and/or when the bottle was first opened.
The new labels also comply with CLP requirements. The guaranteed specification of the product appears on the label if space allows. Otherwise current VWR specifications can be found on vwr.com.

13. How to handle chemicals safely?
Before handling hazardous chemicals, review the safety data sheet and then carry out a risk assessment.